Amid FDA Concerns, HTL-Strefa Inc. Offers a Reliable and Safe Alternative to Chinese Supplied Needles & Syringes With European-Produced Sicura™ Passive Safety Needle
DropSafe® Sicura™ is the first and only fully passive safety needle in North America. Sicura™ designed to eliminate needlestick injuries, providing a safe injection experience, and is distributed by HTL-Strefa Inc., an MTD company.
JERSEY CITY, N.J., March 27, 2024 (Newswire.com) - In response to the recent safety communications issued by the U.S. Food and Drug Administration (FDA) regarding concerns over plastic syringes and safety needles manufactured in China, HTL-Strefa Inc. is proud to offer healthcare providers, facilities, and consumers a safe and dependable alternative. Our FDA-cleared, European-produced passive safety needle, DropSafe® Sicura™, represents the pinnacle of safety, reliability, and performance, fully aligning with the highest standards of patient care and safety.
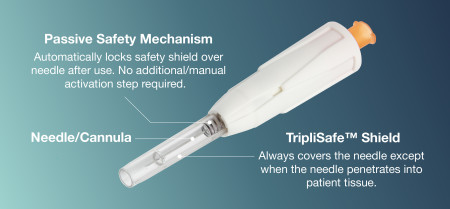
HTL-Strefa Inc.'s new DropSafe® Sicura™ passive safety needle is engineered and manufactured in our own European facility, adhering to stringent quality control measures. Our products are designed with both the healthcare provider and patient in mind, ensuring ease of use, minimal risk of needlestick injuries, and optimal safety during and after administration.
Launched in the U.S. and Canada in 2023, DropSafe® Sicura™ Passive Safety Needle is unique in its design to eliminate needlestick injuries providing a safe injection experience. The needle is protected by a transparent shield that automatically locks after injection, requiring no additional activation step by the HCP post-injection.
**Addressing Current Challenges with Superior Quality and Capacity**
In light of the FDA's evaluation of quality and performance issues associated with certain plastic syringes and safety needles, HTL-Strefa Inc. recognizes the urgent need for reliable medical supplies. Our DropSafe® Sicura™ passive safety needle is not only a testament to our commitment to safety, but also an assurance of our capability to meet the increased demand without compromising on quality or performance.
Carl Ward, General Manager of HTL-Strefa, Inc., emphasized the company's readiness, stating, "HTL-Strefa Inc. has the capacity and the infrastructure to support additional safety needle demand in the U.S. and Canada with the launch of DropSafe® Sicura™. We stand ready to assist healthcare systems in transitioning to safer, more reliable alternatives without any disruption to patient care."
** Committed to Supporting Healthcare Providers Through a Legacy of Excellence and Innovation**
With a history of excellence and innovation in medical technology, HTL-Strefa Inc. and its parent MTD Group have served the healthcare industry for over three decades. Our commitment to enhancing the safety and efficiency of clinical care has driven us to develop advanced technology, services, and solutions that support healthcare professionals and improve patient outcomes.
For more information on HTL-Strefa Inc's. Sicura™ Passive Safety Needle and our commitment to quality and safety, please visit https://dropsafe.info/products/dropsafe-sicura-passive-safety-needle-drug-delivery/ or contact our customer support team at 1-877-660-1900.
**About HTL-Strefa Inc. and MTD Group**
HTL-Strefa Inc. and its parent MTD Group are leading global medical technology companies specializing in the development, manufacture, and distribution of high-quality medical devices, including passive safety needles, lancets, and pen needles. With a strong focus on innovation, safety, and customer satisfaction, HTL-Strefa Inc. and MTD serve healthcare professionals and patients globally, aiming to improve healthcare outcomes and enhance the quality of life. For more information on HTL-Strefa Inc. and MTD Group, please visit https://mtdglobal.com/north-america/ or connect with us on LinkedIn at https://www.linkedin.com/company/htlstrefainc/
**Contact Information:**
Emily Pupa
HTL-Strefa Inc.
113 Towne Lake Pkwy, Suite 120
Woodstock, GA 30188, USA
Source: HTL-Strefa Inc.
